Lyophilization Cycle Scale-Up and Transfer
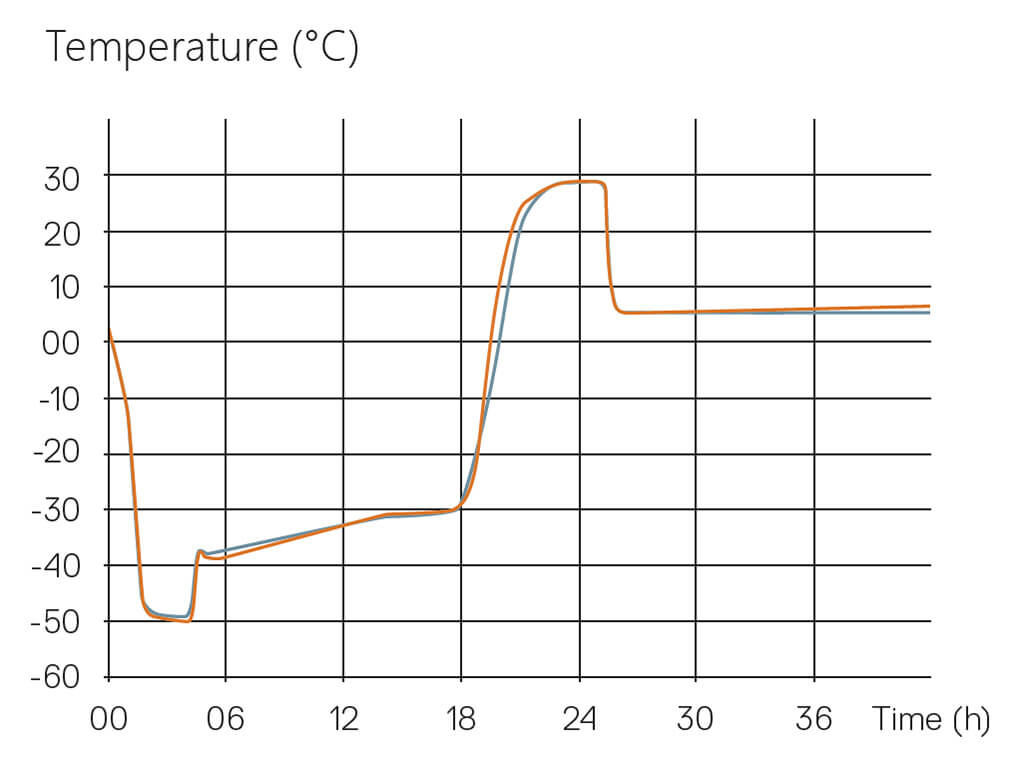
Lyophilization Cycle Scale-Up and Transfer require reproducing the initial product temperature profile onto the target freeze-dryer. By application of the Tempris system, the product temperature differences arising during the transfer can be quantified and compensated by process adaptation, and the characterization of equipment effects can be improved.
During the scale-up of a freeze-dried product, many processing parameters that affect the product quality can change. This is not only due to differences in the equipment setup and performance but also includes environmental factors. The same systematic differences can also occur when transferring a freeze-dried product to a different production freeze-dryer. To obtain a comparable product and achieve consistency in the product quality attributes, the reproduction of the product temperature profile in the target freeze dryer is of paramount importance.
Important Factors
Some important factors that can lead to changes in the freezing and drying properties of a product during scale-up and transfer are:
- Thermal radiation effects transfer additional heat to the product and lead to elevated product temperatures in exposed vials. The extent of radiation depends on the characteristics of the equipment surfaces, such as chamber door and walls. Typically, radiation effects are higher in small-scale units, which leads to shorter drying time and stronger gradients within the batch.
- Shelf temperature heating and cooling rates are often limited in manufacturing equipment compared to modern laboratory scale units, and larger gradients in shelf surface temperature can arise during fast ramping. In addition, potential worst-case positions (hot and cold spots) need to be determined and considered for each freeze-dryer.
- The refrigeration performance of the condenser system in the production freeze-dryer is often a limiting factor in the transfer and scale-up of lyophilization processes. The commonly higher condenser surface temperature compared to small-scale equipment leads to longer process times and can limit the mass transfer rate due to loss of pressure control.
- Another freeze-dryer-related factor that impacts the product temperature profile and the product quality is the type of sensor used for pressure control (e.g., Capacitance Manometer and Pirani). If a different sensor type is applied in the target freeze-dryer, this can lead to a change in absolute pressure of more than 30% and significantly impact the drying properties.
- The traditionally applied product temperature sensors in laboratory freeze drying (thin wire thermocouples) and manufacturing (PT-100) are poorly comparable. The operation principles of these sensors are completely different, as thermocouples provide a point measurement at the bottom of the product. In contrast, the larger PT-100 sensors record average temperatures over the cake. The resulting product temperature profiles are poorly comparable and cannot be used to investigate scale differences or process adaptation systematically.
- The loading system and product setup on the shelves also influence the product temperature profile and the drying properties. This is especially relevant for changes from tray loading to automatic loading systems, the transition from vials on trays to vials on shelves, or if specific heat transfer racks (standard for dual-chamber syringes) are varied over different scales.
- In addition to differences between the freeze-dryers, the effect of environmental conditions needs to be considered. While process development is primarily conducted in non-classified laboratories, aseptic manufacture entails Class A clean room conditions with minimal particle content. This leads to a higher degree of supercooling before nucleation in production and correspondingly smaller ice crystals, higher product resistance, higher product temperature, and prolonged primary drying time.
Lyophilization Cycle Scale-up and Transfer with Tempris Technology
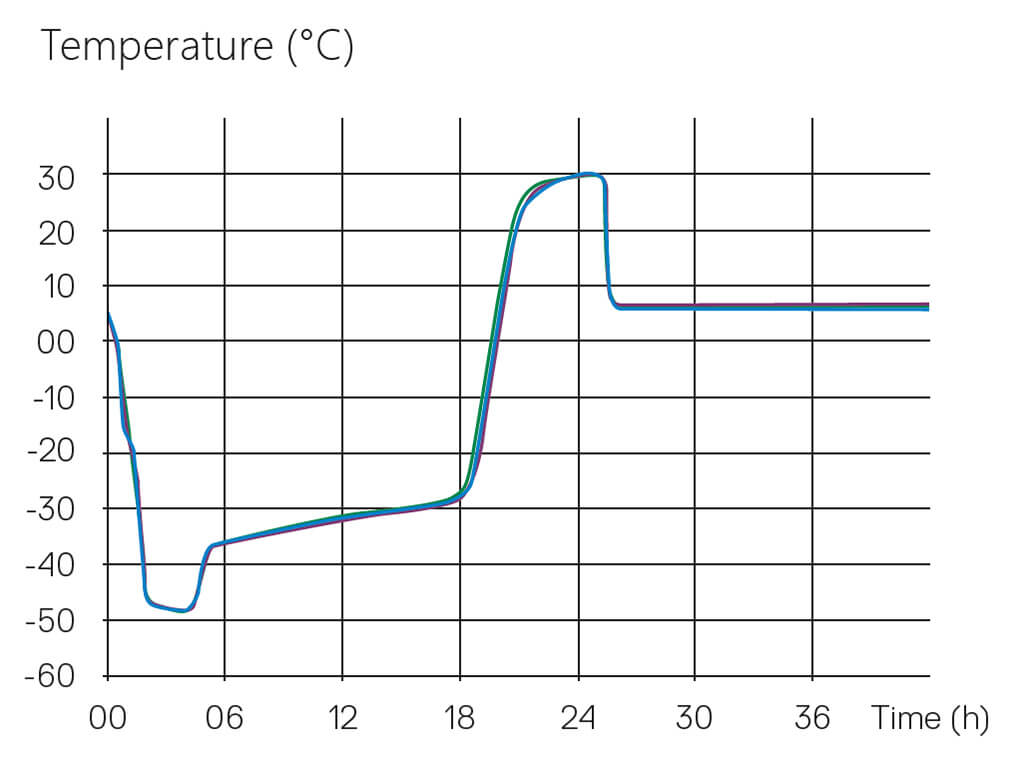
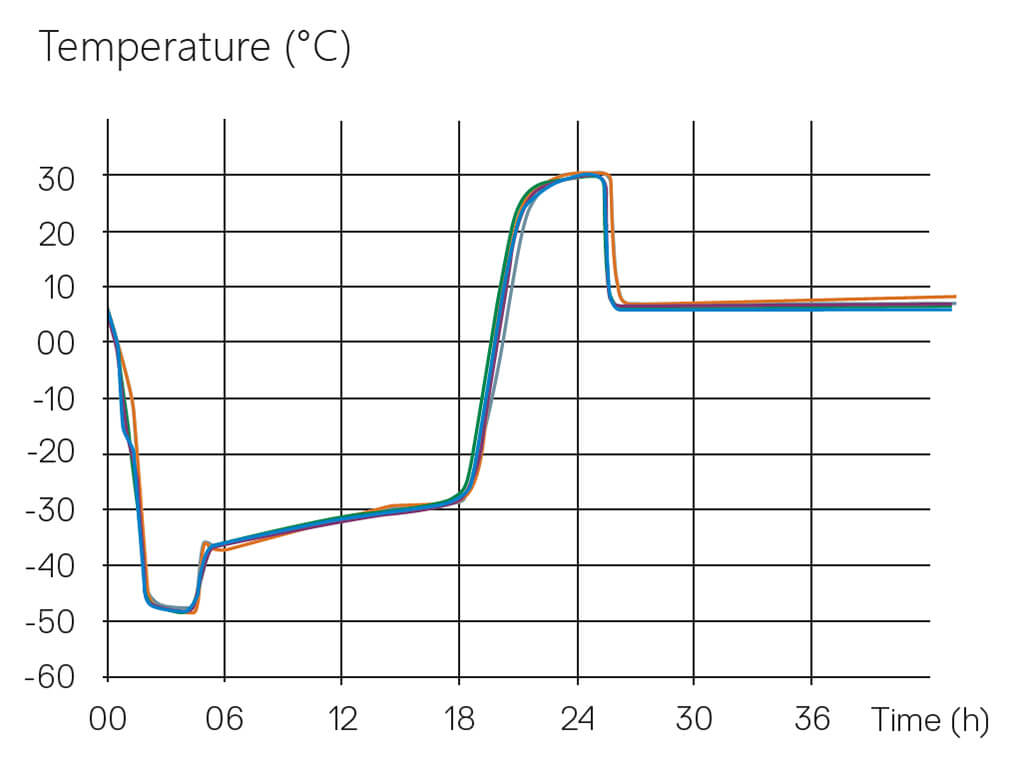
The Tempris technology, designed explicitly for Lyophilization Cycle Scale-Up and Transfer, is applicable on all scales of freeze-dryers, even in production units with automatic loading and unloading systems, and thereby allows comparison of consistent product temperature profiles over the scale-up and transfer process. A process developed in a laboratory scale can directly correlate to subsequent pilot scale batches used for clinical supply, up to validation and routine batches in manufacturing scale equipment. This way, Tempris sensors supply data for the most important critical quality attribute, product temperature, throughout the product life cycle with identical measurement technology. In addition, the product temperature profiles allow early detection of equipment-related differences between freeze-dryers, including the extension of freezing and primary drying times, which require compensation. This way, process conditions can be more easily adapted to achieve an acceptable product with a high degree of assurance in product quality.