PAT Tool for Pharma 4.0
Freeze-drying: Real-time monitoring of product temperature
In modern freeze-dryers, the product temperature, the most important quality parameter, is no longer measured directly. Reasons for this are automatic loading systems and the manual placement of wired thermocouples. Without direct temperature control, manufacturers must, therefore, move to more conservative, longer freeze-drying cycles. The PAT tool ‘Tempris’ offers an alternative by enabling pharmaceutical manufacturers to monitor product temperature in real-time during the freeze-drying process, ensuring the targeted product quality.
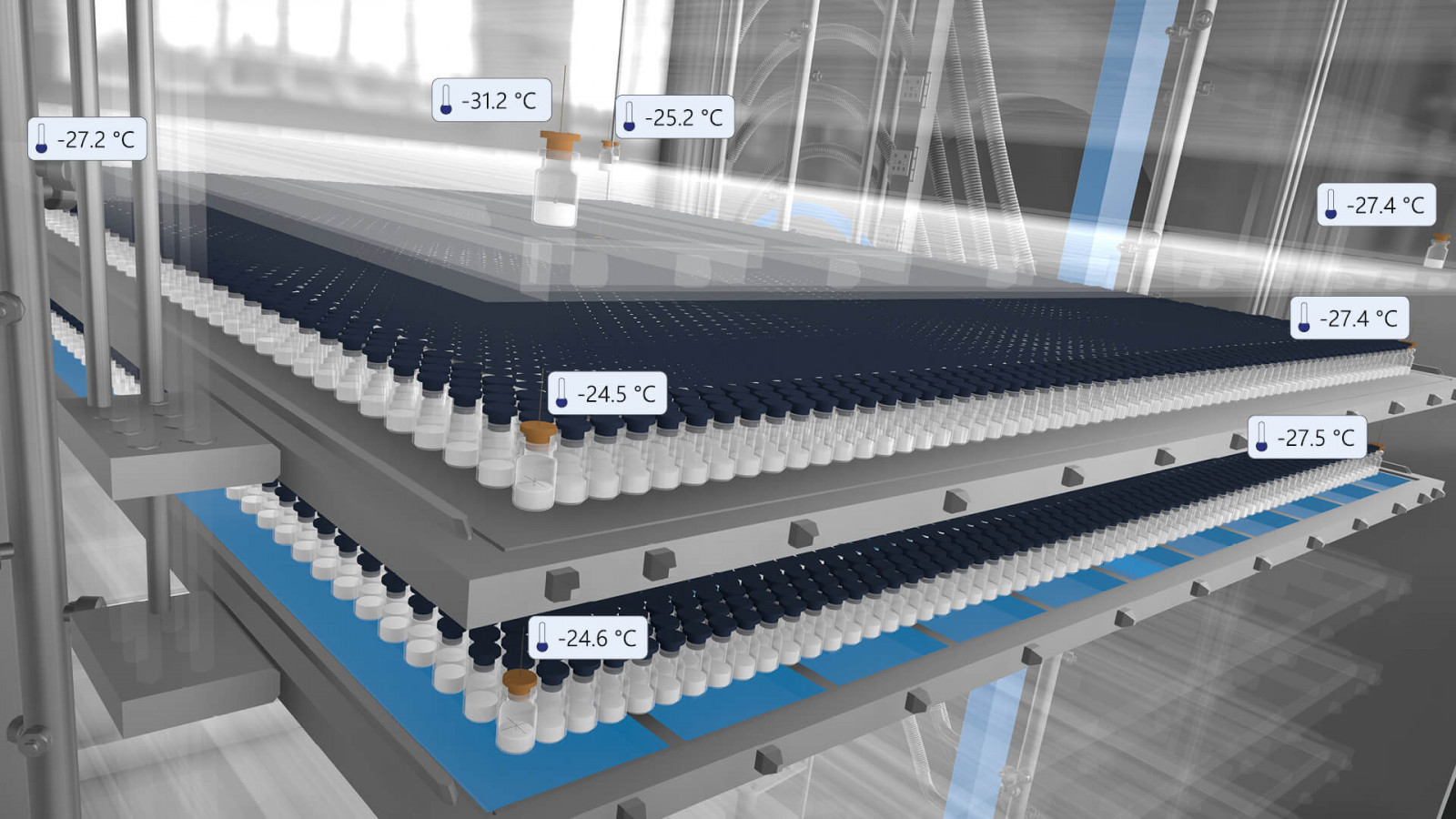
The traditional method of analog temperature monitoring during freeze-drying is error-prone and can cause costly losses. Optimization is, therefore, critical. A common problem is the lack of adjustment of the end point of primary and secondary drying. A premature transition to secondary drying can lead to cake collapse or melting. Therefore, it is important to accurately determine the end point of primary drying.
Tempris has developed a battery-free and wireless process analysis system for real-time monitoring and control of product temperature for drug lyophilization. With Tempris, a system is now available to determine product temperature and drying endpoints much more precisely.
Lyophilization with the PAT tool ‘Tempris’
The traditional development of a lyo process results in a predetermined program that must be applied under all circumstances. The product temperature (Tp) is the most important parameter of a lyophilization process but is rarely measured. Tp is influenced by several factors and cannot be controlled directly.
Tempris offers a solution by determining the product temperature at critical positions, the so-called ‘Hot and Cold Spots’ (HCS). This allows the recipe program to be adjusted and ensures that the product temperature always remains within the developed design space. Tempris measures the product temperature in real-time and provides crucial information for continuous process stability.
Dr. Andrea Weiland-Waibel reports that this approach was already successfully used in an approval process 2018. Since then, this wireless temperature measurement has been applied to every market batch as a key element for ongoing process verification. Since 2018, Explicat Pharma has produced more than 150 batches without any product losses, despite deviations, as Tp could be verified at any time according to the approval.
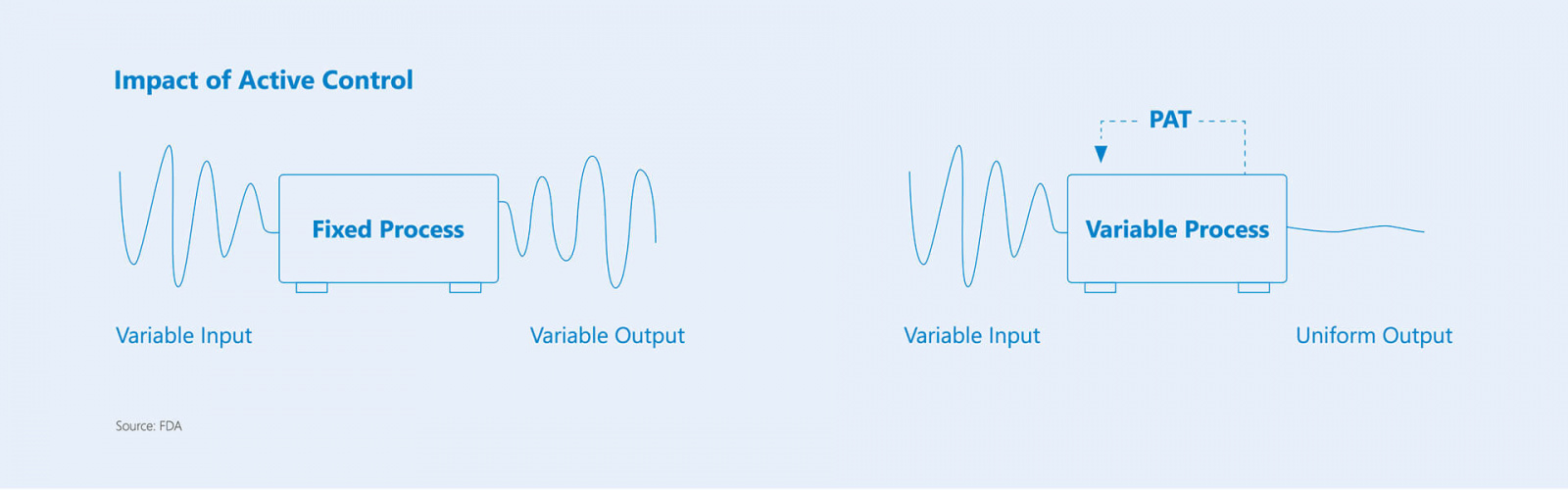
The Tempris PAT tool offers enormous potential for pharmaceutical manufacturers and CMOs. Tempris technology makes dynamic process adaptation possible, leveraging new opportunities in drug approval and thus enabling cost and time advantages. Product temperature deviation approval has never been easier.
Tempris: How the system works
No electrical cable, no batteries, unlimited data storage, and real-time measurement—the Tempris measurement system provides measurement data in real-time for storage or further processing in the SCADA system.
The technology is based on a sensor with oscillating quartz whose resonant frequency changes with temperature and is transmitted by radio. The Tempris sensor obtains its energy exclusively from the radio field generated by antennas in the freeze-drying chamber. The antennas transmit the data to the communication unit, which displays the signals as measured values in the HMI.
Tempris complies with GMP, 21 CFR Part 11 and GAMP 5 and can be retrofitted in any freeze-dryer. It provides unmatched comparability of temperature data from development to production. The FDA has approved Tempris as a PAT tool. Measurement accuracy is well below the required standards. The wireless sensors can be pre-sterilized and placed in vials in a fully automated process.
Tempris: From the lab to production
Tempris allows the product temperature profile developed in the laboratory to be scaled to all freeze-dryer geometries. It allows the assessment of the most important critical product parameter over the entire product life cycle. Freeze-drying plant-specific differences between freeze-dryers can be identified early and process conditions optimized to obtain a high-quality product.
Note: At IFPAC 2023, USA the question was raised, what actually is the advantage of PAT tools compared to today’s usual process validation, which has to be proven only once a year? The answer was: “A PAT tool ensures process stability”.
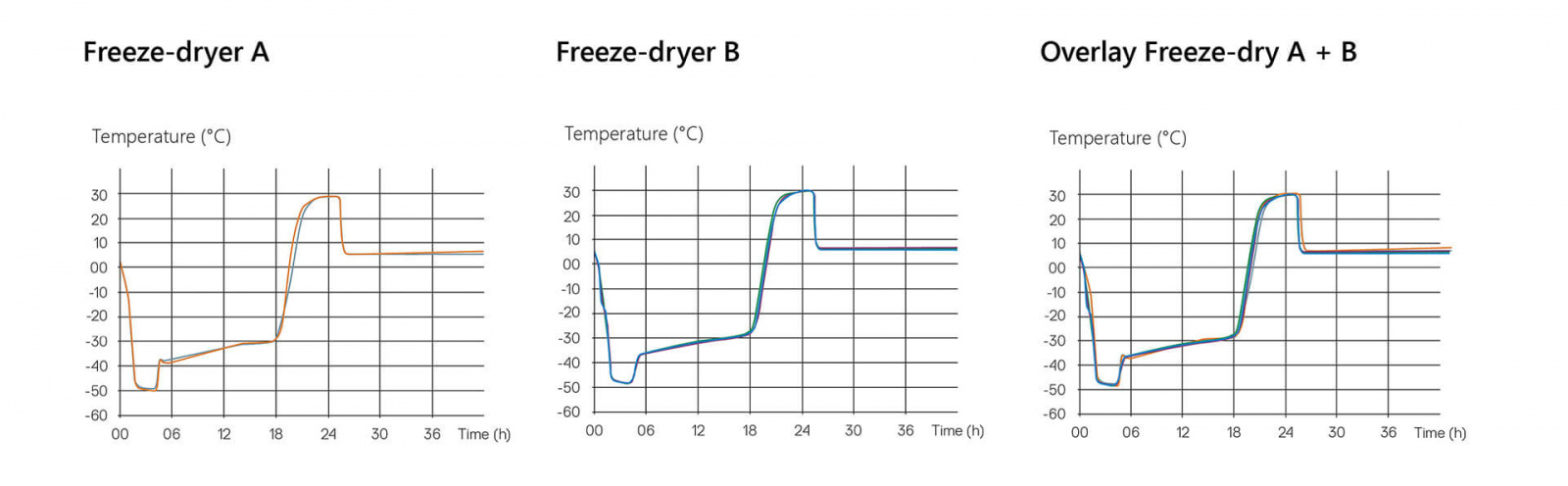
Transfer of Lyo cycle from freeze-dryer A (24 m²) to freeze-dryer B (33 m²). The Lyo cycle temperature profiles from different runs on both freeze-dryers are superimposed to show that the Lyo cycles are equivalent. No further evaluation of the upper and lower temperature limits is required.
Conclusion: A PAT tool for more stable processes
Tempris monitors and documents in real-time the product temperature during lyophilization, enabling more precise control of the freeze-drying process.
Pharmaceutical companies can use Tempris technology to operate their freeze-dryers more agilely, thereby significantly shortening the time-to-market of their products with the PAT tool.
Last but not least, this is important: If entire batches sometimes have to be discarded with the previous manufacturing practice, this is not only a financial loss. It is also important that this material is not available on the market—Tempris, therefore, offers the opportunity to expand production without having to expand the freeze-drying plant itself.
Another point: If Tempris as a PAT tool ensures more stable processes, this increases the productivity of the freeze-drying plant, and last but not least, the system improves the resilience of the freeze-drying plant – deviations/disruptions in the process flow are largely leveled out by Tempris.
What Pfizer says about the Tempris concept
“Recently, we conducted large-scale trials for one of our vaccine candidates using Tempris to ensure the robustness of our process on a large scale. We were able to demonstrate that the product temperature profiles for both the edge and center vials were within the design space, which was confirmed by the robustness study in the lab. This gives us confidence that the commercial process will produce a product with the desired quality – supporting our regulatory submission.”
Pfizer Inc, Pharmaceutical R&D
Holzkirchen, March 14th, 2024 | Author: Hans-Jürgen Bittermann
